Alcohol Nomenclature:Alcohols are just like other
substituents. There isn't anything real special about them. I'm assuming you've read all
the sections before this, so I can breeze through this section.
Molecules with alcohols are called -ols. For instance  is methanol. (methane - ane + ol = methanol).
is methanol. (methane - ane + ol = methanol).
Among alcohols, there are primary, secondary, and tertiary
alcohols.

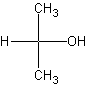
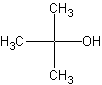
The structure on the left is the primary alcohol. Primary
alcohol means that the -OH is attached to a carbon group which has NONE or ONE other
carbon attached to it.
Secondary alcohols, as demonstrated by the middle structure, has an
-OH group attached to a carbon which is attached to two carbons. It is also called a sec-alcohol.
Tertiary alcohols, the right structure, is an alcohol with the -OH
group attached to a carbon with connections to three other carbons. Tert-alcohols,
as they are called, are very bulky. The significance of these comes later on.
If you want to know how it stands in terms of functional group priority, hit this link.
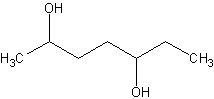
Name this
This is 2,5-heptadiol. No cis/trans becuase it's an alkane. Alkanes have free rotation.