Nomenclature of AlkynesAlkynes are named similarly to
alkenes. Much like alkenes, you change the ending of the molecule name to designate a
triple bond. Instead of the -ane or the -ene, you use -yne. You number the carbon to the
lowest possible carbon number.
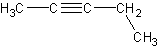
The above molecule is called 2-pentyne.
Alkynes have the lowest priority (other than alkanes, which are THE lowest). This means
that when you have both a double bond and triple bond, the double bond gets the
priority in numbering. Let's look at this molecule:
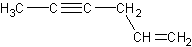
This molecule is called 1-penten-4-yne.
The simplest possible alkyne is the two carbon triple bond, which would be called
ethyne. However, in keeping with "historical" names, the name
"acetylene" is popular. This isn't anything that you can deduce, it's one of the
random names that organic chemists like to use. So, remember that this molecule,
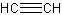
is called acetylene (although ethyne is still a good name to use).
To name alkynes as substituent groups, such as this molecule:
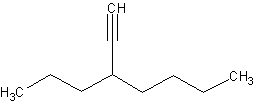
First, count the longest parent chain. The longest parent chain is an octane. The
substituent, located on carbon four, is an ethyne group. To name alkynes as substituents,
you take the name, ethyne, and make it ethynyl. If the substituent was a three carbon
chain with a triple bond, it would be called propynyl.