Amine Nomenclature:Naming an amine is simliar to
naming an alcohol. You just take the parent chain (for instance, hexane), drop the -e, and
add -amine (hence, you would get hexanamine). Try this one:
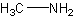
Yes, that's methanamine. Very easy. Remember tertiary, secondary, and primary
structures? Well, in amines, it is important to note which kind of amine you have, because
it affects the nomenclature.
Now, when a hydrogen on the amine group (-NH2) is replaced with something else, you use
N- to designate that it's a substituent of the amine group itself. Let's look at
an example:
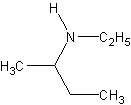
The parent chain is the propane group. The amine is off carbon 2, so we have the name
2-butanamine. However, the amine group has had a hydrogen removed and an ethyl added. So,
the naming would be N-ethyl-2-butanamine. If both the hydrogens have been
removed, you do the N,N- prefix.
The only time amines have a prefix (amino-) is when a higher functional group exists on
the molecule. Alcohols, ketones, aldehydes, and carboxylic acid groups all have higher
priority, so when those are present, the -amine suffix becomes an amino- prefix. Here is
an example:
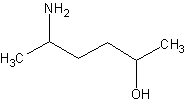
The parent chain is a hexane. Now, normally, if the -OH group wasn't there, this would
be called hexanamine. However, the -OH has a higher priority, so this molecule becomes
5-aminohexan-2-ol.
Amine groups on benzenes are called anilines. Other than that, there is normal
numbering. Try this molecule:
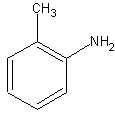
The amine group on the benzene makes this an aniline. The methyl group is ortho to the
amine group. So, we get the name o-methylaniline.
One last exercise:
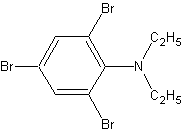
This last molecule will tie in everything we've learned. Ok, the amine group on the
benzene gives us the parent name of aniline. There are three bromine groups on there, so
we'll have a 2,4,6-tribromo prefix. (Notice that when you have this many substituents, you
won't get the positions mixed up, so you don't need ortho, para, or meta). The hydrogens
on the amine group have been replaced with ethyl- groups, so we'll have a N,N-diethyl
prefix as well. The final answer?
N,N-diethyl-2,4,6,-tribromoaniline.
Man, are you a chemist nerd yet, or what? You're naming these complicated molecules. :)