Aromatics:Aromatics, which smell, are one of the more
important chapters in biochemistry.
What is an aromatic molecule?
Aromatic compounds in organic chemistry are those which smell. They contain conjugated
rings of carbons. However, not all conjugated rings are considered aromatic. To determine
which compounds are aromatic, follow the Hückel Rule.
The Hückel rule states that an aromatic 4n + 2 pi electrons will be aromatic, if n is
a positive integer. This means that cyclic planar molecules with 2, 6, 10, 14, 18, 22,
26...etc. pi electrons will be aromatic. Let's take a look.
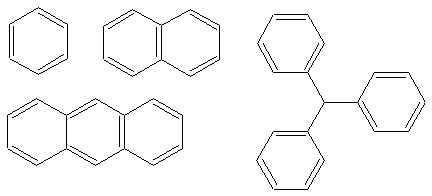
These are all aromatics. Remember that each double bond counts for two pi electrons.
Let's take a look at the top left molecule. This molecule has 3 double bonds. 3 x 2 = 6 pi
electrons. This fits in with Hückel's rule. You can try the calculations for all the
other ones; they all work. The following are NOT aromatic.
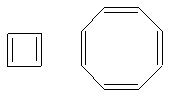
Notice how the left one only has 4 pi electrons (not enough!) and the right onw has 8
pi electrons (sorry!). So, using Hückel's rule, you can figure out almost all the
aromatic compounds.
Benzene
Benzene is symmetrical, planar, hexagonal strcture that boils at 80 C and melts
at 5 C.
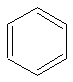
Benzene is the simplest of the aromatics (n=1 for Hückel's rule). Although you may see
benzene written like that, benzene actually exists as two different compounds.
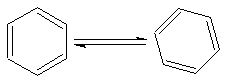
Kekulé structures for benzene
The benzene ring is constantly changing its form because of resonance. You can see the
two structures that are possible for benzene.
Benzene Substituents
Two substituents that involve the benzene ring are phenyl- and benzyl- groups.
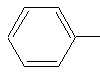
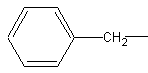
The molecule on the left is the phenyl- group and the one on the right is the benzyl-
group. Aromatic groups are sometimes written Ar- (which is short for aryl) while R-
represents an alkyl group. So if you saw someting written as Ar-CH3, that would
be a methylbenzene (or phenylmethane).