Cyclic Ethers:Hopefully you've read the tutorial on
acyclic ethers. If not, go read it.
Cyclic ethers look like this:
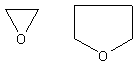
They are oxygen containing rings. Since these are very easy to name, they are also
referred to by their common names rather than the IUPAC names (although IUPAC naming is
still common). Historically, these O-containing rings have been called epoxides.
The IUPAC has given the name oxirane to these molecules. Which one you
use is strictly up to you. (A random note, the top right molecule is known as
tetrahydrofuran (THF))
To name these molecules historically, take the longest chain, name it as you would name
an alkene, and then add "oxide" to the end. For instance, for this molecule:
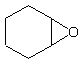
The longest parent chain is a cyclic hexane, or cyclohexane. A cyclo-carbon molecule
with double bonds would be called cyclohexene. So, now we add oxide and we get the name
cyclohexene oxide. (Note the space).
Cis/Trans conformations
Cis/trans conformations are possibly in cyclic ethers, since the bonds are rigid (no
free rotation).
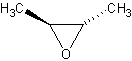
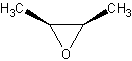
The molecule on the left is trans-2-Butene oxide, while the one of the left is
cis-2-Butene oxide.