U.S. National Chemistry Olympiad: 1993 Local
Test
Go to Answers
1. Which diagram is the best representation for a mixture of hydrogen and helium at 25
°C and 1 atm?
2. Which combination of 1 M solutions will produce a visible reaction when mixed?
Solution Mixtures:
I. HCl and Pb(NO3)2
II. HCl and NaOH
III. HCl and Na2CO3
(A) I and II only
(B) I and III only
(C) II and III only
(D) I, II, III
3. Which physical property would be most useful for determining whether two samples of
metal are the same metal?
(A) mass
(B) volume
(C) color
(D) density
4. Which technique could be used to separate a homogeneous mixture?
Techniques:
I. chromatography
II. distillation
III. filtration
(A) I only
(B) III only
(C) I and II only
(D) I, II, III
5. How many of these elements are liquids at 25 °C and 1 atm?
Elements:
mercury
phosphorus
radon
(A) 0
(B) 1
(C) 2
(D) 3
6. What is the mole fraction of methanol, CH3OH, in a solution of methanol
and water that contains 50 g of each?
| Substance |
Molar Mass |
| CH3OH |
32 g mol¯1 |
| H2O |
18 g mol¯1 |
(A) 0.18
(B) 0.36
(C) 0.50
(D) 0.64
7. Ammonia can be reacted with oxygen to form nitrogen(II) oxide and water according to
the unbalanced equation
_NH3 + _O2 ---> _ NO + _H2O
When this equation is balanced with the simplest set of whole number coefficients, the
coefficients are
(A) 1,1,1,1
(B) 2,1,2,3
(C) 2,5,2,3
(D) 4,5,4,6
8. A lab manual directs a student to measure a piece of ribbon 5.00 cm long and
calculate its mass from the mass of 100.00 cm of the ribbon (0.985 g). Why is this
approach superior to simply cutting a 5.00 cm piece of ribbon and determining its mass on
an electronic milligram balance?
(A) Magnesium ribbon can corrode the balance pan as it is being weighed
(B) Such a small piece of ribbon will not give a readout on a milligram balance
(C) The length can be measured more rapidly than the mass
(D) Measuring its length gives a more precise mass
9. What should be done to prepare 500.0 mL of a 0.200 M solution of NaCl?
The Molar Mass of NaCl is 58.45 g mol¯1
(A) 11.7 g of NaCl should be dissolved in 500.0 mL of H2O
(B) 11.7 g of NaCl should be dissolved in sufficient H2O to give a total volume
of 500.0 mL
(C) 5.85 g of NaCl should be dissolved in sufficient H2O to give a total volume
of 500.0 mL
(D) 2.92 g of NaCl should be dissolved in 500.0 mL of H2O
10. Equal masses of O2 and N2 are reacted according to this
equation:
O2 + N2 ---> 2 NO
Which statement is true?
(A) O2 is the limiting reagent and N2 is present in excess
(B) N2 is the limiting reagent and O2 is present in excess
(C) all of the O2 and N2 react and neither is in excess
(D) Nothing can be said about the limiting reagent
11. When a 0.817 g sample of a copper oxide is heated with excess hydrogen gas, 0.187 g
of water is formed. What is the apparent formula of the copper oxide?
(A) CuO
(B) Cu2O
(C) Cu2O3
(D) CuO2
12. A reaction in which DS and DH
are positive is expected to be
(A) nonspontaneous under all conditions
(B) spontaneous under all conditions
(C) more spontaneous at low temperatures
(D) more spontaneous at high temperatures
13. Which process or reaction has a positive DH?
(A) H2O(l) ---> H2O(s)
(B) 2 CH3OH(l) + 3 O2(g) ----> 2 CO2(g) + 4 H2O(l)
(C) CO2(s) ---> CO2(g)
(D) 2 Na(s) + Cl2(g) ---> 2 NaCl (s)
14. For which process or reaction is DS positive at 25 °C?
(A) 2 NO2(g) ---> N2O4(g)
(B) CO2(g) ---> CO2(s)
(C) CH4(g) + 2 O2(g) ---> CO2(g) + 2 H2O(l)
(D) 2 SO3(g) --- > 2 SO2(g) + O2(g)
15. For the reaction
H2(g) + I2(s) ---> 2 HI(g) DHrxn
= 53.0 kJ
What will be the value of DHrxn (in kJ) for this
reaction ?
HI(g) ---> 1/2 H2(g) + 1/2 I2(s)
(A) 26.5
(B) 7.3
(C) -26.5
(D) -53.0
16. For the reaction of hydrogen peroxide and iodide ion in acid solution, represented
by the equation:
H2O2 + 3I¯ + 2H+ ---> I3¯
+ 2 H2O
these kinetic data are gathered:
| |
Initial Concentrations, M |
| |
H2O2 |
I¯ |
H+ |
Initial Rate, M s¯1 |
| Exp. 1 |
0.010 |
0.010 |
0.00050 |
1.15 x 10¯6 |
| Exp. 2 |
0.020 |
0.010 |
0.00050 |
2.30 x 10¯6 |
| Exp. 3 |
0.020 |
0.020 |
0.00050 |
4.60 x 10¯6 |
| Exp. 4 |
0.020 |
0.020 |
0.00100 |
4.60 x 10¯6 |
What is the rate law for the reaction?
(A) Rate = k [H2O2] [I¯]
(B) Rate = k [H2O2] [I¯] [H+]
(C) Rate = k [H2O2]2 [I¯]
(D) Rate = k [H2O2] [I¯]2
17. A small increase in temperature often causes a large increase in the rate of a
chemical reaction. This effect is best attributed to
(A) a decrease in the activation energy of the reaction
(B) more frequent collisions at the higher temperature
(C) the occurrence of more collisions with the needed energy
(D) different reaction pathways at the higher temperature
18. Zinc metal reacts with excess HCl according to the equation:
Zn(s) + 2H+(aq) + 2Cl¯(aq) ----> Zn2+(aq) +
2Cl¯(aq) + H2(g)
Which change will increase the rate of evolution of H2?
Changes in Reaction Conditions
I. using zinc dust in place of chunks
II. using 2 M HCl in place of 1 M HCl
III. using 200 mL of 1 M HCl in place of 100 mL
(A) I only
(B) I and II only
(C) II and III only
(D) I, II, and III
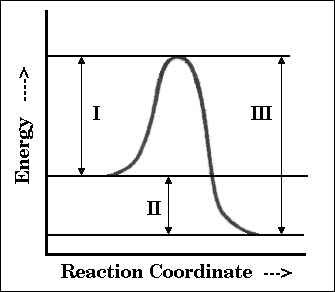
19. Which of the indicated quantities in the reaction diagram will be affected by the
addition of a catalyst?
(A) I only
(B) II only
(C) I and II only
(D) I and III only
20. Gases can be compressed more easily than liquids because
(A) gas molecules are larger than liquid molecules
(B) molecules move more slowly in gases than in liquids
(C) average intermolecular distances are greater in gases
(D) intermolecular forces increase as gas molecules are brought closer together
21. A mixture of N2 (0.40 mol), O2 (0.50 mol) and Ne (0.30 mol)
exerts a pressure of 740 mmHg. What would be the pressure of the N2 alone at
the same temparature in the same container (in mmHg)?
(A) 740
(B) 400
(C) 333
(D) 247
22. At pressures below its triple point a substance can exist
(A) only as a gas.
(B) only as a solid.
(C) as a solid or a liquid.
(D) as a solid or a gas.
23. When a beaker of pure water is boiling vigorously, the bubbles that rise to the
surface are composed primarily of
(A) air.
(B) hydrogen.
(C) hydrogen and oxygen.
(D) water vapor.
24. If the pressure of an ideal gas sample is tripled while the temperature is halved,
which expression best describes the final volume?
(A) Vfinal = 0.06 x Vinitial
(B) Vfinal = 0.167 x Vinitial
(C) Vfinal = 0.667 x Vinitial
(D) Vfinal = 1.5 x Vinitial
25. A compound boils at 45 °C, does not conduct electricity appreciably as a pure
liquid but produces an electrically-conducting solution when dissolved in water. When this
substance is cooled below its freezing point (16 °C), what type of solid is formed?
(A) ionic
(B) metallic
(C) molecular
(D) network covalent
26. Chemical equilibrium is defined as the point when
(A) all reaction ceases.
(B) products and reactants are formed at equal rates.
(C) reactants are all converted to products.
(D) reactants and products are present at equal concentrations.
27. Which is true for a reaction with an equilibrium constant of 3.0 x 10¯15?
(A) The reactants are favored.
(B) The products are favored.
(C) Equilibrium will be reached slowly.
(D) Equilibrium will be reached rapidly.
28. The exothermic formation of ClF3 is represented by the equation:
Cl2(g) + 3 F2(g) <===> 2 ClF3(g) DH = -329 kJ
Which change will increase the quatity of ClF3 in an equilibrium mixture of
Cl2, F2, and ClF3?
(A) increasing the temperature
(B) removing Cl2
(C) increasing the volume of the container
(D) adding F2
29. Which will be the same for solutions of 0.50 M NH3 and 0.50 M NaOH?
Solution Variables:
I. concentration of OH¯ ion
II. volume of 0.20 M HCl required for neutralization
(A) I only
(B) both I and II
(C) II only
(D) neither I nor II
30. The solubility of which salt will be increased the most in 1 M HCl (relative to its
solubility in H2O)?
(A) BaCO3
(B) PbCl2
(B) NaNO3
(D) CuSO4
31. Which is the strongest acid?
(A) HF
(B) HNO2
(C) H2SO4
(D) H3PO4
32. What is the pH of 20.0 mL of 0.050 M HCl?
(A) 1.30
(B) 1.70
(C) 2.60
(D) 3.00
33. Which is amphotheric (behaves either as an acid or as a base) in aqueous solution?
(A) HClO3
(B) Ca(OCl)2
(C) NaOH
(D) Zn(OH)2
34. Which will yield the most acidic solution when 0.10 mol is mixed with 1 L of H2O?
(A) CaO
(B) Al2O3
(C) SiO2
(D) SO2
35. When a particular aqueous solution is diluted by a factor of ten with H2O,
the pH increases by one pH unit. This solution most likely contains a
(A) weak acid
(B) strong base
(C) strong acid
(D) buffer
36. Which species can act as both an oxidizing agent and a reducing agent?
(A) F2
(B) Cs
(C) ClO4¯
(D) SO32¯
37. Which is an oxidation-reduction reaction?
(A) Cu + 4 HNO3 ---> Cu(NO3)2 + 2 NO2 +
2 H2O
(B) AgNO3 + NaBr ---> AgBr + NaNO3
(C) Na2O + H2O ---> 2 NaOH
(D) NH3 + HF ---> NH4F
38. When this equation is balanced correctly, what is the coefficient for I2?
__BrO3¯ + __I¯ + __H+ ---> __Br¯ + __I2
+ __H2O
(A) 1
(B) 2
(C) 3
(D) 6
39. Which species contains the most neutrons?
(A) 26-Fe-59
(B) 29-Cu-61
(C) 30-Zn-61
(D) (30-Zn-60)2+
40. Which type of radiation changes both the atomic number and mass number of the
emiting atom?
(A) alpha
(B) beta
(C) gamma
(D) X-ray
41. Which group of elements is listed in order of increasing boiling point?
(A) lithium, sodium, potassium
(B) chlorine, bromine, iodine
(C) sulfur, chlorine, argon
(D) zinc, cadmium, mercury
42. The formula for the oxide of an element, X, is X2O3. Which is
the most likely formula for another compund of this element?
(A) XF6
(B) XH4
(C) X2Cl3
(D) X(NO3)3
43. Which element is the best electrical conductor?
(A) Al
(B) Si
(C) P
(D) Cl
44. Which is true about the relative sizes of these species?
(A) Na < Na+; F < F¯
(B) Na > Na+; F > F¯
(C) Na > Na+; F < F¯
(D) Na < Na+; F > F¯
45. Which atomic property generally increases down the periodic table but decreases
from left to right across it?
(A) ionization energy
(B) atomic number
(C) principal quantum number
(D) atomic radius
46. How many valence electrons are present in the C2O42¯
ion?
(A) 44
(B) 42
(C) 34
(D) 30
47. Which element combinations will form ionic compounds?
Element Combinations
I. Ca (Z = 20) and Ti (Z = 22)
II. Si (Z = 14) and Br (Z = 35)
III. Mg (Z = 12) and Cl (Z = 17)
(A) II only
(B) III only
(C) II and III
(D) I, II, and III
48. Which species is best represented by more than one Lewis structure (that is,
exhibits resonance)?
(A) NH4+
(B) O3
(C) CO
(D) SF4
49. Which molecule has the shortest bond length?
(A) N2
(B) O2
(C) F2
(D) Cl2
50. Which molecule will have the largest F-X-F bond angle?
(A) OF2
(B) NF3
(C) BF3
(D) CF4
51. Which piece of glassware should be used to measure 8.70 mL of a solution?
(A) 20-mL graduated cylinder
(B) 25-mL volumetric flask
(C) 50-mL buret
(D) 100-mL beaker
52. During flame tests, which set of metal salts gives the colors red, yellow, green
and violet, respectively?
(A) Sr, Na, Ba, K
(B) Na, Ba, Sr, K
(C) Sr, K, Ba, Na
(D) K, NA, Ba, Sr
53. The density of an insoluble object is determined by weighing it on a balance, then
submerging it in a graduated cylinder to find its volume. Based on the data collected, to
how many significant figures should the density be reported?
| Density Data |
| mass |
23.92 g |
| initial H2O volume |
66 mL |
| final H2O volume |
72 mL |
(A) 1
(B) 2
(C) 3
(D) 4
54. When betitol, C6H12O4, is burned with excess
oxygen to form carbon dioxide and water, how many moles of oxygen are needed for each mole
of betitol?
(A) 1
(B) 6
(C) 7
(D) 9
55. A 2.70 g sample of an unknown hydrocarbon was burned in excess oxygen to form 88.0
g of carbon dioxide and 27.0 g of water. What is a possible molecular formula for the
hydrocarbon?
(A) CH4
(B) C2H2
(C) C4H3
(D) C4H6
56. When 500.0 mL of 1.0 M LaCl3 and 3.0 M NaCl are mixed, what is the
molarity of the chloride ion?
(A) 4.0 M
(B) 3.0 M
(C) 2.0 M
(D) 1.5 M
57. How is each property affected when a nonvolatile solute is added to a pure solvent?
| |
Vapor pressure |
freezing point |
boiling point |
| (A) |
increases |
increases |
increases |
| (B) |
increases |
decreases |
increases |
| (C) |
decreases |
decreases |
decreases |
| (D) |
decreases |
decreases |
increases |
58. How many joules are required to raise the temperature of 35.0 mL of liquid mercury
from 25 °C to 38 °C?
| Properties of liquid mercury |
| Specific heat |
0.139 J g¯1 °C¯1 |
| Density |
13.6 g mL¯1 |
(A) 6.6 x 101
(B) 2.5 x 102
(C) 8.6 x 102
(D) 6.2 x 103
59. The reaction between gaseous ammonia and oxygen is given by the equation:
4 NH3(g) + 7 O2(g) ---> 4 NO2(g) + 6
H2O(l)
DH = -1397.8 kJ
What is the standard enthalpy of formation of NH3(g) [in kJ mol¯1],
given these standard enthalpies of formation?
| Standard Enthalpies of Formation |
| NO2(g) |
33.18 kJ mol¯1 |
| H2O(l) |
-285.18 kJ mol¯1 |
(A) -45.1
(B) -63.1
(C) -184.4
(D) -252.6
60. The reaction: 2 ICl + H2 ---> 2 HCl + I2 has been proposed
to occur by this mechanism:
H2 + ICl --- HCl + HI (slow)
HI + ICl --- HCl + I2 (fast)
Which rate law best agrees with this information?
(A) Rate = k [H2]
(B) Rate = k [ICl]2
(C) Rate = k [H2] [ICl]2
(D) Rate = k [H2] [ICl]
61. Which gas had an average molecular velocity that is 4 times as great as that of
sulfur dioxide?
The Molar Mass of SO2 is 64 g mol¯1
(A) H2 (M = 2 g mol¯1)
(B) He (M = 4 g mol¯1)
(C) CH4 (M = 16 g mol¯1)
(D) O2 (M = 32 g mol¯1)
62. For the reaction;
2 NO(g) + O2(g) <===> 2 NO2(g)
the equilibrium concentrations at a certain temperature are found to be [NO] = 0.10 M,
[O2] = 0.20 M, [NO2] = 0.30 M. The value of the equilibrium constant
for this reaction at this temperature is
(A) 1.5
(B) 15
(C) 45
(D) 150
63. A solution of Na2CO3 is added slowly to a solution initially
containing equal concentrations of Ba2+, Cd2+, Fe2+, and
Zn2+ ions. Which compound will precipitate first?
| Solubility Product Constant |
| BaCO3 |
2.6 x 10¯9 |
| FeCO3 |
3 x 10¯11 |
| CdCO3 |
6 x 10¯12 |
| ZnCO3 |
1 x 10¯10 |
(A) BaCO3
(B) FeCO3
(C) CdCO3
(D) ZnCO3
64. According to the equations;
H2CO3 + H2O <===> H3O+
+ HCO3¯
HCO3¯ + H2O <===> H3O+
+ CO32¯
what is the conjugate base of HCO3-?
(A) H2CO3
(B) CO32¯
(C) H2O
(D) H3O+
65. What is the [OH¯] in the final solution prepared by mixing 20.0 mL of 0.050 M HCl
with 30.0 mL of 0.10 M of Ba(OH)2?
(A) 0.12 M
(B) 0.10 M
(C) 0.40 M
(D) 0.0050 M
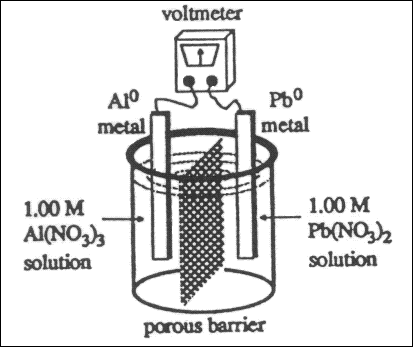
66. What voltage will be produced by the electrochemical cell?
| Reduction Potentials |
| Pb2+ + 2 e¯ ---> Pb |
-0.13 V |
| Al3+ + 3 e¯ ---> Al |
-1.68 V |
(A) 2.97V
(B) 1.55V
(C) -1.81V
(D) -2.97V
67. How many grams of cobalt metal will be deposited when a solution of cobalt(II)
chloride is electrolyzed with a current of 10. amperes for 109 minutes?
(A) 0.66
(B) 4.0
(C) 20
(D) 40
68. Which element will have the greatest number of unpaired electrons?
(A) Co (Z = 27)
(B) Ni (Z = 28)
(C) Cu (Z = 29)
(D) Ga (Z = 31)
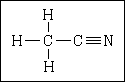
69. What is the hybridization of the carbon that is bonded to the nitrogen?
| (A) sp |
 |
| (B) sp2 |
| (C) sp3 |
| (D) dsp3 |
70. Which molecule is polar?
(A) CO2
(B) NH3
(C) SiCl4
(D) PF5
|