Chemical Equilibrium
What is Chemical Equilibrium?
As the name equilibrium suggests, it is some kind of balance. In this case it
is a chemical rate balance.chemical equilibrium = occurs when opposing reactions
are going at the same rate.
This concept is actually very simple. Let's look at an example of a chemical
equilibrium.

If we put some H and I molecules into a container and wait a bit, at the beginning
there will be no HI present at all. But after a while (the H and I are colliding together,
thus reacting) the amount of HI will increase, this will result in a decrease in the
amount of H and I.
Now let's look at what happens when two HI molecules collide to react.

We will get the reverse reaction. The HI breaks apart to form H and I again.
This is a never-ending process, each reaction will occur over and over again forever.
Let's recap what will happen when we mix H and I together:
1. The H and I will react to form HI.
2. The HI will form rapidly since there are a lot of H and I colliding
together.
3. The concentration of H and I will decrease.
4. The HI will start to collide (reacting) with each other to form H
and I.
5. This will be slow at first, because of the low concentration of HI.
6. HI concentration will increase with time, the reaction rate will be
faster also.
7. At some point, all the HI, H and I concentration will reach a level
where the rate of both reaction are the same. This is what we call a chemical
equilibrium.
How do we write equilibrium equations?
Instead of writing out two equations (the forward and reverse reactions) every time,
we can just write it as:

Equilibrium Stoichiometry Problems
Let's see how we can apply what we just learned to solve some chemistry problems.
- key point: If we put two known amounts of reactants in a container and let
it reach chemical equilibrium, and if we know one of the substance's concentration at the
equilibrium. We then determine ALL the amounts of all the other substances.
Example: In a container, we have 0.0930 mole of NO and 0.0652 mole of Br2
reacting until equilibrium.
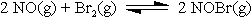
At the equilibrium there is 0.0612 mole of NOBr. Use this information to
determine the moles for each of the substance at equilibrium.
fTo solve this problem, it would be much easier if we make a table, this
table is extremely helpful in solving these type of problems.
fStep 1: Let's enter into the table all the data we have.
|
2 NO (g) |
+ |
Br2 |
 |
2 NOBr(g) |
| Beginning |
0.0920 moles |
|
0.0652 moles |
|
0 moles |
| the Change |
- |
|
- |
|
- |
| Equilibrium |
- |
|
- |
|
0.0612 moles |
Step 2: We can figure out the Change in NOBr just by looking at the table. In
the beginning we have 0 moles then at the equilibrium we have 0.0612 moles. The change
would be +0.0612 moles. Let's put this information into the table.
|
2 NO (g) |
+ |
Br2 |
 |
2 NOBr(g) |
| Beginning |
0.0920 moles |
|
0.0652 moles |
|
0 moles |
| the Change |
- |
|
- |
|
+0.0612 moles |
| Equilibrium |
- |
|
- |
|
0.0612 moles |
Step 3: If we know that it takes 2 moles NO to form 2 moles of NOBr, and the
NOBr changed by +0.0612 moles, thus we can reason that NO will be reduced by the same
amount (-0.0612 moles). What about Br2? It takes 1/2 molar amount of Br2
to form 1 mole of NOBr, thus, if NOBr increased by +0.0612 moles, Br2 had to be
decreased by Half of that amount (-0.0306 moles).
|
2 NO (g) |
+ |
Br2 |
 |
2 NOBr(g) |
| Beginning |
0.0920 moles |
|
0.0652 moles |
|
0 moles |
| the Change |
-0.0612 moles |
|
-0.0306 moles |
|
+0.0612 moles |
| Equilibrium |
- |
|
- |
|
0.0612 moles |
Step 4: Calculate the answer.
For NO it is 0.0920 moles - 0.0612 moles = answer for NO
For Br2 it is 0.0652 moles - 0.0306 moles = answer for Br2
|
2 NO (g) |
+ |
Br2 |
 |
2 NOBr(g) |
| Beginning |
0.0920 moles |
|
0.0652 moles |
|
0 moles |
| the Change |
-0.0612 moles |
|
-0.0306 moles |
|
+0.0612 moles |
| Equilibrium |
0.0318 moles |
|
0.0346 moles |
|
0.0612 moles |
Step 5: The final answer.
At equilibrium the amount of NO is 0.0318 moles, and for Br2 it is 0.0346 moles
The Equilibrium Constant>>
Le Chatelier's Principle>>