Conjugated Dienes TutorialSince you already know how
to name alkenes, this tutorial will focus more on the
concepts of dienes.
There are three types of conjugated dienes:
- Isolated - these are double bonds that are isolated

- Cumulative - these are double bonds that follow one another
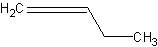
- Conjugated - these are double bonds that are separated by one single
bond.

As you will read later, conjugated dienes are very important.
Delocalization- existence of p electron
cloud extends over four carbons (in a conjugated diene), opposed to two in a normal -ene.
This is important because the delocalization means that the carbon has more electrons to
share amongst itself, which lowers energy and makes the molecule more stable.
Resonance theory - states whenever a molecule or ion can be
represented by two or more structures that differ only in the position of the electrons,
there are three implications: (I shamelessly stole these off of my organic chem book)
- There are multiple structures to represent the same molecule
- The actual structure is a mixture (hybrid) of the different possible structures
- The molecule or ion is considerable more stable (that is, of lower energy) than would be
expected based on any single structure
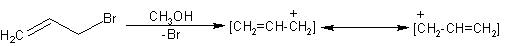
This is a reaction where the bromine is removed from an alkene. The removal of bromine
creates a carbocation (since the bromine stole the electron pair that originally kept the
carbon neutral). A carbocation is a carbon with a positive charge. As the image above
demonstrates, the p bonds from the neighboring carbon can
"shift" over to the carbocation. However, this leaves the other carbon with a
positive charge. Eventually, you will add something to get rid of that charge. This is
resonance. Later on, in the aromatics chapter, you will see more examples of resonance.